Directed evolution of a Bacillus-derived glycosyltransferase for enhanced glycosylation efficiency
Abstract
The Bacillus-derived glycosyltransferase (GT) BmmGT1 exhibited broad substrate flexibility, especially towards sugar acceptors,showing a great potential in natural products diversification.However, compared to the natural sugar acceptor macrolactin A (MLN A), the conversion rates of BmmGT1 towards other acceptors are much lower.Herein, the catalytic activities of BmmGT1 towards sugar acceptors was enhanced via directed evolution. A small library of variant BmmGT1 with mutated binding sites mutants were generated and screened using a 3-acetyl-7-hydroxycoumarin-based ultraviolet spectrophotometry screening method. Two mutants (M79F and M79W) were obtained,showing ~2.1-fold higher glucosylation activities towards 3-acetyl-7-hydroxycoumarin than the wild-type BmmGT1. Their catalytic activities towards other sugar acceptors were further tested, and the glucosylation activities of M79W was identified with ~3.6-fold and ~1.2-fold increase towards MLN A and piericidin A,respectively.Our study would provide guidance for engineering of other natural-product GTs via directed evolution.
Introduction
Glycosylation of natural products can improve the parent compound’s pharmacological properties, specificity, even the molecular mechanism of action[1-3]. Hence, sugar moieties are essential structural units in many clinical drugs, includingantibiotics[4], anticancer drugs[5], antifungal[6] and antiparasitic[7] agents. Installation of the sugar moieties of natural products is usually carried out by glycosyltransferases (GTs)[1-3]. They can catalyze glycosyl transfer reaction from an activated donor, in most cases a nucleoside diphosphate (NDP) sugar, onto specific acceptor molecules, forming glycosidic bonds[1-3].Based on their protein structures, GTs could be clarified into twomajor structural folds defined as GT-A and GT-B. Most of the natural products GTs belong to the GT-B foldfamily, which is characterized by two distinctβ/α/β Rossmann type domains with a connecting linker[3, 8]. Bacterial GTs can usually recognize a wide variety of sugar donors and acceptors, making them powerful tools for the glycodiversification of natural products[9-11].
Directed evolution involves random introduction of mutations into genes and screening and thus can be applied to improve enzyme properties[12].Directed evolution has been proven to be a powerful and efficient tool for improving enzyme properties, including catalytic activity, molecular stability, substrate selectivity, stereoselectivity, and tolerance of high substrate or product concentrations[13-15]. It has been efficiently used in engineering of P450s, decarboxylases,etc [12]. Owing to the lack of high-throughput screening and selection method, reports of directed evolution of GTs are limited. For example, only the oleandomycin glycosyltransferase OleD from Streptomyces antibioticus was engineered by error-prone PCRand screened using a fluorescent acceptor 4-methyl-7-hydroxycoumarin, which led to the identification of a triple-mutant with broader sugar donor promiscuity[16]; and the glycosyltransferase BLC from Bacillus licheniformis was engineeredvia directed evolution, generating mutants with enhanced catalytic activities towards avermectin[17].Giventhe importance of sugar moieties in natural products, it is greatly desired of directed evolution of GTs to effectivelygeneratediverse bioactiveglycosides.
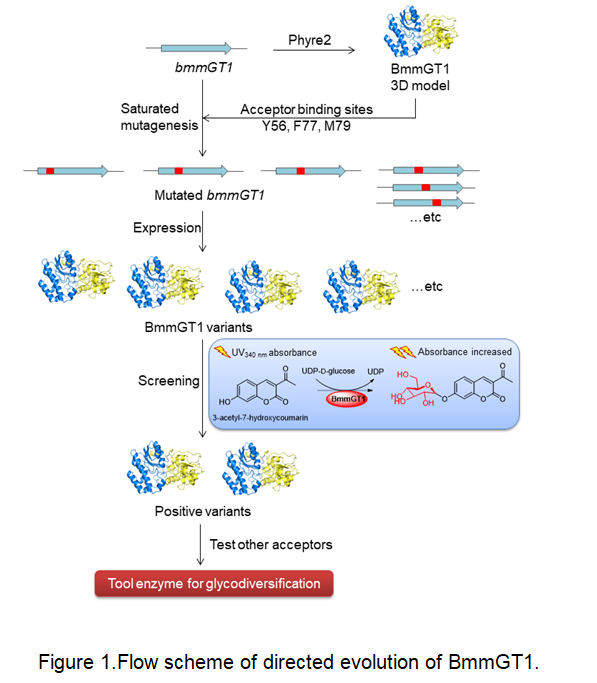
Previously, we identified a macrolide GT gene, bmmGT1, from Bacillus methylotrophicus B-9987, and demonstrated that it was responsible for the O-glycosylation of macrolactins (24-membered macrolide) and bacillaenes (polyunsaturated enamines)[18]. Notably, BmmGT1 displays broad substrate flexibility, especially toward sugar acceptors, transferring glucose to piericidin A, 3,4-dichloroaniline and 3,4-dichlorothiophenol, generating O-, N- or S-glycosides[19, 20].However, the conversion rates of these reactions were much lower than the rate with the natural acceptor macrolactin A (MLN A). Thus, improving BmmGT1 enzyme activity towards different sugar acceptors has great potential for its application in these glycosides production.
Coumarins are a class of naturally-occurring phenolic compounds featuring anα-pyrone ring fused with a benzene ring[21] (Fig S1). 7-hydroxycoumarin (umbelliferone) (Fig S1) exhibits antinociceptive and anti-inflammatory activities and their glycosides have been found to possess neuroprotective effects with antidiabetic and antioxidative activities[22-24]. 7-hydroxycoumarins possess a large π-π conjugated system with electron-rich and charge transfer properties, making them fluorescent sensors for biological activities;conversely substitutionsat 7-OH lose the fluorescent properties[25]. Thus 7-hydroxycoumarin and its derivatives have been developed as a simple high-throughput screen method for detection of β-glucuronidase, CYP enzyme and glycosynthase assays[16, 25].
Herein, we developed a 3-acetyl-7-hydroxycoumarin-based ultraviolet spectrophotometry screening method. Saturation mutation of the sugar binding sites in BmmGT1 was performed and mutants with enhanced glucosylation activities towards 3-acetyl-7-hydroxycoumarin were screened. Furthermore, the catalytic activities of these mutants towards alternative sugar acceptors (MLN A, piericidin A and 3,4-dichloroaniline)were assessed (Fig1).