iDEC 2021 | OUC-Marine Drugs
Directed evolution of a Bacillus-derived glycosyltransferase for enhanced glycosylation efficiency
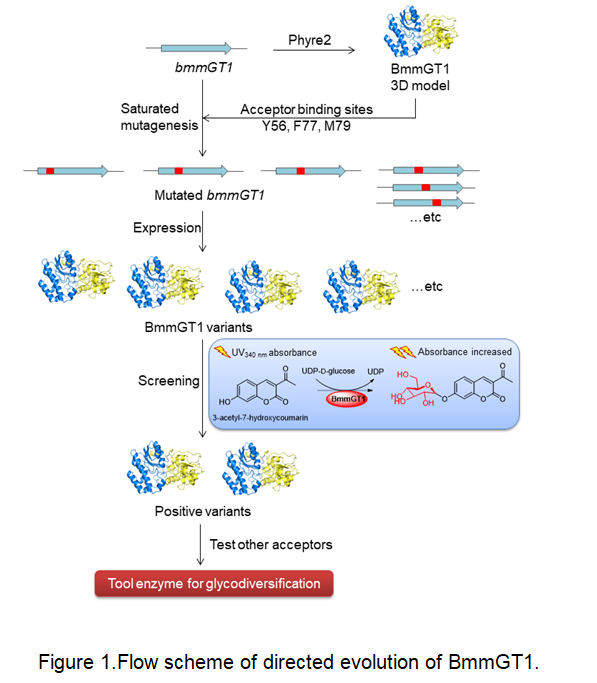
The Bacillus-derived glycosyltransferase (GT) BmmGT1 exhibited broad substrate flexibility, especially towards sugar acceptors, showing a great potential in natural products diversification. However, compared to the natural sugar acceptor macrolactin A (MLN A), the conversion rates of BmmGT1 towards other acceptors are much lower. Herein, the catalytic activities of BmmGT1 towards sugar acceptors was enhanced via directed evolution. A small library of variant BmmGT1 with mutated binding sites mutants were generated and screened using a 3-acetyl-7-hydroxycoumarin-based ultraviolet spectrophotometry screening method. Two mutants (M79F and M79W) were obtained, showing ~2.1-fold higher glucosylation activities towards 3-acetyl-7-hydroxycoumarin than the wild-type BmmGT1. Their catalytic activities towards other sugar acceptors were further tested, and the glucosylation activities of M79W was identified with ~3.6-fold and ~1.2-fold increase towards MLN A and piericidin A, respectively. Our study would provide guidance for engineering of other natural-product GTs via directed evolution.
Our Team
Team OUC-Marine_Drugs is based on the Ocean University of China,Laboratory for Marine Drugs and Bioproducts of Qingdao National Laboratory for Marine Science and Technology. Our team focuses on enhancing glycosyltransferase efficiency.
